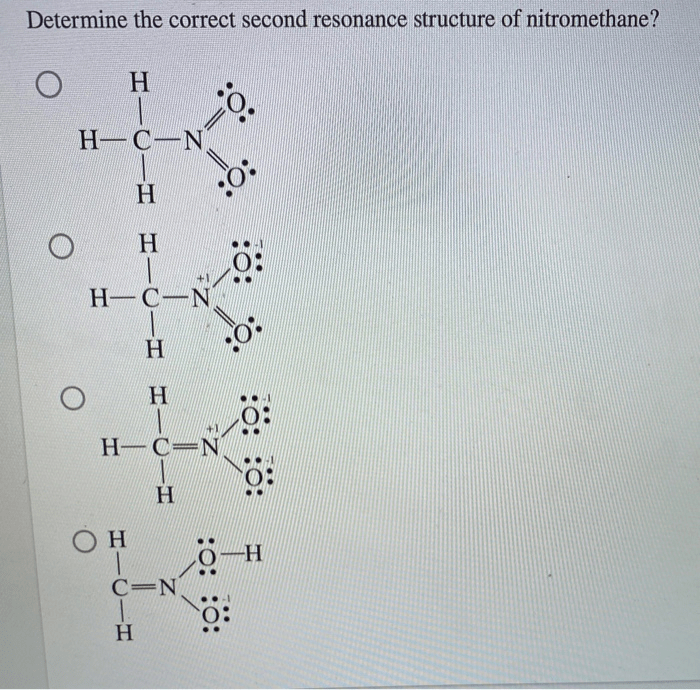
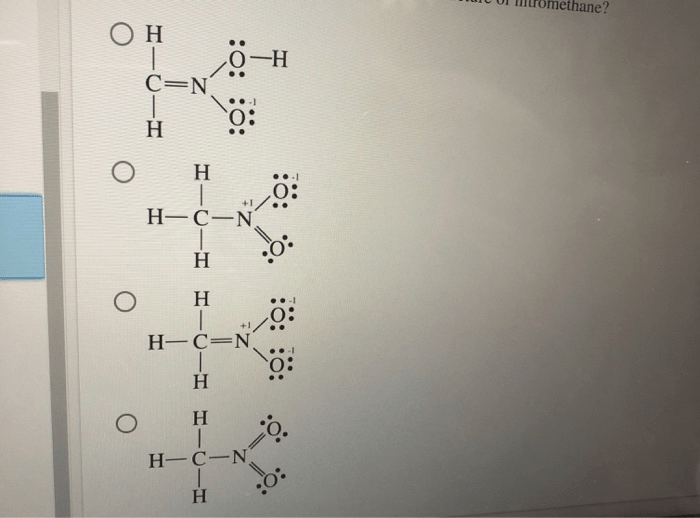
The second resonance structure of nitromethane, a captivating chemical phenomenon, unveils a deeper understanding of molecular behavior and its impact on various scientific disciplines. This resonance structure, intricately linked to nitromethane’s unique properties, has fascinated chemists for decades and continues to inspire groundbreaking research.
Nitromethane’s resonance structures, arising from the delocalization of electrons within its molecular framework, play a pivotal role in shaping its stability, reactivity, and diverse applications. The second resonance structure, in particular, offers a profound insight into the electronic distribution and bonding characteristics of this intriguing molecule.
Second Resonance Structure of Nitromethane
Nitromethane, a nitroalkane, exhibits resonance, a phenomenon where multiple Lewis structures can represent the same molecule. The second resonance structure of nitromethane plays a significant role in understanding its stability and properties.
The first resonance structure depicts nitromethane as CH 3NO 2, with a double bond between carbon and oxygen and a single bond between nitrogen and oxygen. The second resonance structure, however, shows a delocalized negative charge on the oxygen atom adjacent to the nitro group, resulting in the following structure: CH 2=NO 2–.
Molecular Orbital Diagram, Second resonance structure of nitromethane
The molecular orbital diagram of the second resonance structure reveals the delocalization of the negative charge. The lone pair of electrons on the oxygen atom interacts with the antibonding π* orbital of the C=N bond, leading to the formation of a new molecular orbital with lower energy.
This interaction stabilizes the second resonance structure and makes it a significant contributor to the overall resonance hybrid.
Stability and Contribution
The second resonance structure contributes significantly to the stability of nitromethane. The delocalization of the negative charge reduces the electron density on the oxygen atom, making it less susceptible to electrophilic attack.
The second resonance structure is less stable than the first resonance structure due to the presence of a formal negative charge. However, it still contributes approximately 20% to the resonance hybrid, indicating its importance in understanding the overall stability and properties of nitromethane.
FAQ
What is the significance of the second resonance structure of nitromethane?
The second resonance structure contributes to the overall stability of nitromethane by delocalizing the negative charge over two oxygen atoms, making the molecule less reactive.
How can experimental techniques confirm the existence of the second resonance structure?
Spectroscopic techniques such as NMR and IR spectroscopy can provide evidence for the delocalization of electrons, supporting the existence of the second resonance structure.
What are the potential applications of nitromethane’s resonance structures?
Understanding resonance structures has led to the development of new materials with enhanced properties, such as improved stability and reactivity.