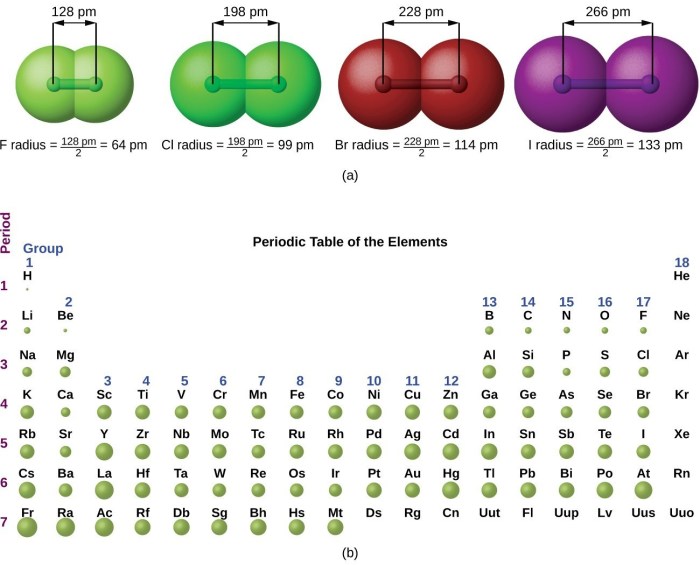
Rank the following atoms in terms of decreasing atomic radius: the quest to understand the fundamental properties of matter begins with the exploration of atomic radii. This intrinsic property, defined as the distance from the nucleus to the outermost electron shell, plays a pivotal role in determining the chemical and physical behavior of elements.
Join us on a journey to unravel the factors influencing atomic radius, uncover the methods employed to rank atoms, and delve into the fascinating trends that emerge from this analysis.
As we embark on this scientific expedition, we will encounter the periodic table, a roadmap that organizes elements based on their atomic number and electron configurations. We will discover how atomic radius varies across periods and groups, revealing the underlying principles that govern the size of atoms.
Factors Affecting Atomic Radius

Atomic radius is influenced by several factors, including:
- Atomic number (Z):As the atomic number increases, the number of protons in the nucleus increases, leading to a stronger electrostatic attraction between the nucleus and the electrons. This attraction pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
- Number of energy levels (n):Electrons occupy different energy levels around the nucleus. As the number of energy levels increases, the electrons are further away from the nucleus, resulting in a larger atomic radius.
- Shielding effect:Inner electrons can shield the outer electrons from the attraction of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outer electrons, allowing them to occupy larger orbitals and resulting in a larger atomic radius.
Trends in Atomic Radius
Atomic radius generally decreases across a period (from left to right) and increases down a group (from top to bottom) in the periodic table.
Across a period:As the atomic number increases, the number of protons in the nucleus increases, leading to a stronger electrostatic attraction between the nucleus and the electrons, resulting in a smaller atomic radius.
Down a group:As the atomic number increases down a group, the number of energy levels also increases, causing the electrons to be further away from the nucleus and resulting in a larger atomic radius.
Methods for Ranking Atoms, Rank the following atoms in terms of decreasing atomic radius
Atoms can be ranked in terms of atomic radius using the following methods:
- Periodic table:The periodic table arranges elements in order of increasing atomic number, which also corresponds to decreasing atomic radius across a period.
- Experimental measurements:Atomic radii can be experimentally determined using techniques such as X-ray diffraction and electron microscopy.
- Theoretical calculations:Atomic radii can also be calculated using quantum mechanical models, which take into account the electrostatic interactions between electrons and the nucleus.
Table of Atomic Radii
| Element Name | Symbol | Atomic Number | Atomic Radius (pm) |
|---|---|---|---|
| Hydrogen | H | 1 | 53 |
| Helium | He | 2 | 31 |
| Lithium | Li | 3 | 155 |
| Beryllium | Be | 4 | 111 |
| Boron | B | 5 | 85 |
Key Questions Answered: Rank The Following Atoms In Terms Of Decreasing Atomic Radius
What is the significance of atomic radius?
Atomic radius is a crucial property that influences various aspects of an element’s behavior, including its chemical reactivity, bonding characteristics, and physical properties such as density and melting point.
How does atomic radius vary across the periodic table?
Atomic radius generally decreases across a period from left to right due to the increase in effective nuclear charge. Moving down a group, atomic radius typically increases as new electron shells are added.